Molecular control of experience-dependent brain rhythms is uncovered (Neuron)
The research team led by Prof. MA Huan has recently published an article titled Gating of hippocampal rhythms and memory by synaptic plasticity in inhibitory interneurons on Neuron online on February 5th. This research uncovered gCaMKII as a mediator of LTPE-I and molecular control of experience-dependent brain rhythms, suggesting that E-I synaptic plasticity plays a gatekeeping role in tuning experience-driven E-I spike transmission, network activity and therefore mnemonic function.
Neuronal activity triggered by experience modifies the strength of excitatory synapses onto inhibitory interneurons (E-I), which in turn exerts powerful control over brain networks by regulating oscillatory neural organization. Such experience-dependent network changes are essential for learning and memory. However, major questions remain about the molecular mechanisms through which these E-I synapses are shaped by experience, and how this process contributes to neural computation.
E-I synaptic input can be dynamically modified. Synaptic plasticity, exemplified by long-term potentiation (LTP), is a fundamental process thought to underlie the long-term storage of information in neural circuits. Although LTP has been studied extensively, the majority of knowledge centers on the potentiation of excitatory synapses onto excitatory neurons (LTPE-E), with far less known about excitatory synaptic potentiation onto inhibitory neurons (LTPE-I). The lack of the knowledge about how LTPE-I is generated at the molecular level has hampered understanding of its function at the network and behavioral levels in vivo.
To address this fundamental question, researchers from the team led by Prof. Ma recorded the extracellular local field potential (LFP) in the hippocampus of freely moving mice. Their results showed that E-I monosynaptic drive is positively correlated with LFP power, particularly at theta and gamma rhythms. This suggests that changes in E-I synaptic input may modulate LFP power.
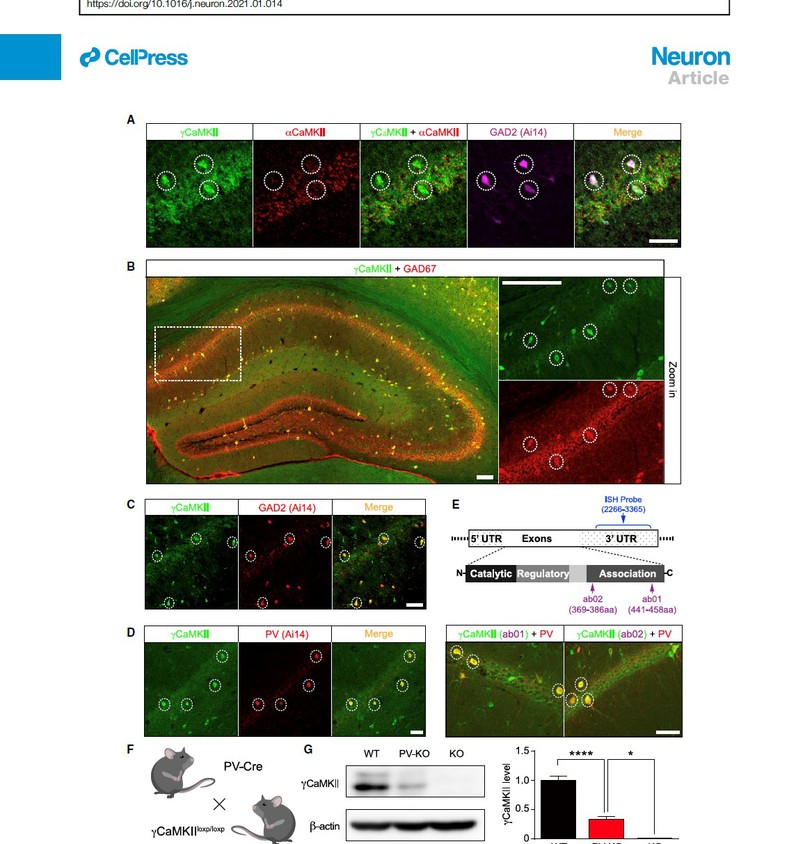
aCaMKII, the key mediator of LTPE-E, is absent from inhibitory interneurons and is not required for LTPE-I. The team of Prof. Ma leveraged recent technical advances, including sensitive RNAscope in situ hybridization (ISH) and immunostaining. They foundgCaMKII, another isoform of CaMKII family, is enriched specifically in GABAergic interneurons, with particular enrichment in PV+ interneurons. They also found gCaMKII PV-KO mice, in which gCaMKII is selectively deleted in PV+ interneurons, showed reduced fear response in contextual CFC test. Their data strongly suggest that gCaMKII in hippocampal PV+ interneurons plays a critical role in hippocampus-dependent long-term memory.
Next, they found gCaMKII PV-KO mice have normal synaptic transmission and LTPE-E but impaired LTPE-I according to the in vitroelectrophysiological experiments. In addition, the phosphorylation level of GluR1, a key step involved in the LTP process, in the PV interneurons of PV-KO mice did not increase after the CFC training. These results indicate that gCaMKII is critical for experience-dependent AMPAR regulation in hippocampal PV+ interneurons.
In order to establish a causal relationship between E-I synaptic drive and oscillation, researchers performed in vitro and in vivo electrophysiological recording. Deleting gCaMKII in PV+ interneurons disrupted E-I spike transmission coupling and experience-driven strengthening in theta and gamma rhythmicity. They suggest that LTPE-I regulates activity-dependent E-I spike transmission coupling, and therefore plays a critical role in experience-dependent rhythms and memory in vivo.
Graduate students HE Xingzhi, LI Jiarui, ZHOU Guangjun and postdoctor YANG Jing from Zhejiang University School of Medicine are co-first authors of this article. Prof. MA Huan from Zhejiang University School of Medicine is the corresponding author. This study is mainly supported by the National Natural Science Foundation of China andthe National Key R&D Program of China.


