Key player in regulatory mechanism for innate immune response uncovered in ZJU
The research team led by WANG Xiaojian, a professor of immunology in the School of Medicine, worked together with the research team led by SUN Jihong from the Department of Radiology in the Sir Run Shaw Shaw Hospital to discover the function of downregulated NDR1 protein kinase in inhibiting innate immune response by initiating an miR146a-STAT1 feedback loop. The findings are published online in the July 17 issue of the journal of Nature Communications.
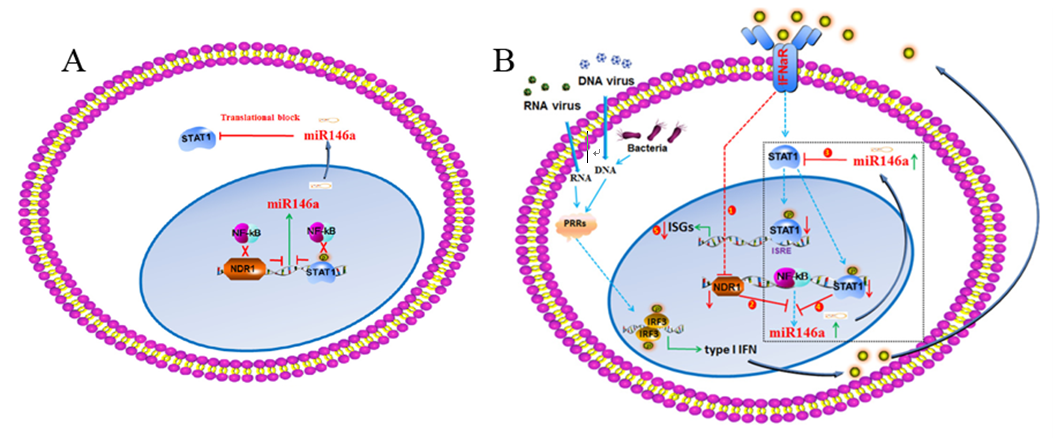
Interferon (IFN)-stimulated genes (ISGs) play crucial roles in the antiviral immune response. However, IFNs also induce negative regulators that attenuate the antiviral response. WANG Xiaojian et al. show that both viral and bacterial invasion downregulate Nuclear Dbf2-related kinase 1 (NDR1) expression via the type I IFN signaling pathway. NDR1 promotes the virus-induced production of type I IFN, proinflammatory cytokines and ISGs in a kinase-independent manner. NDR1 deficiency also renders mice more susceptible to viral and bacterial infections. Mechanistically, NDR1 enhances STAT1 translation by directly binding to the intergenic region of miR146a, thereby inhibiting miR146a expression and liberating STAT1 from miR146a-mediated translational inhibition. Furthermore, STAT1 binds to the miR146a promoter, thus decreasing its expression.
Together, the findings reveal a novel regulator of innate immunity, NDR1, which is downregulated by infection with various viruses and regulates IFN signaling by maintaining STAT1 translation. Given that NDR1 is required for STAT1 expression and HCV clearance in liver cells, NDR1 may be a potential target for the clinical treatment of HCV infection.